The fundamental symmetries and conservation laws are essential building blocks in our understanding of the
physical-chemical world. If, as in the traditional theory of atoms and molecules, only the electromagnetic
interaction is included, the symmetry with respect to space inversion is generally accepted. But if also
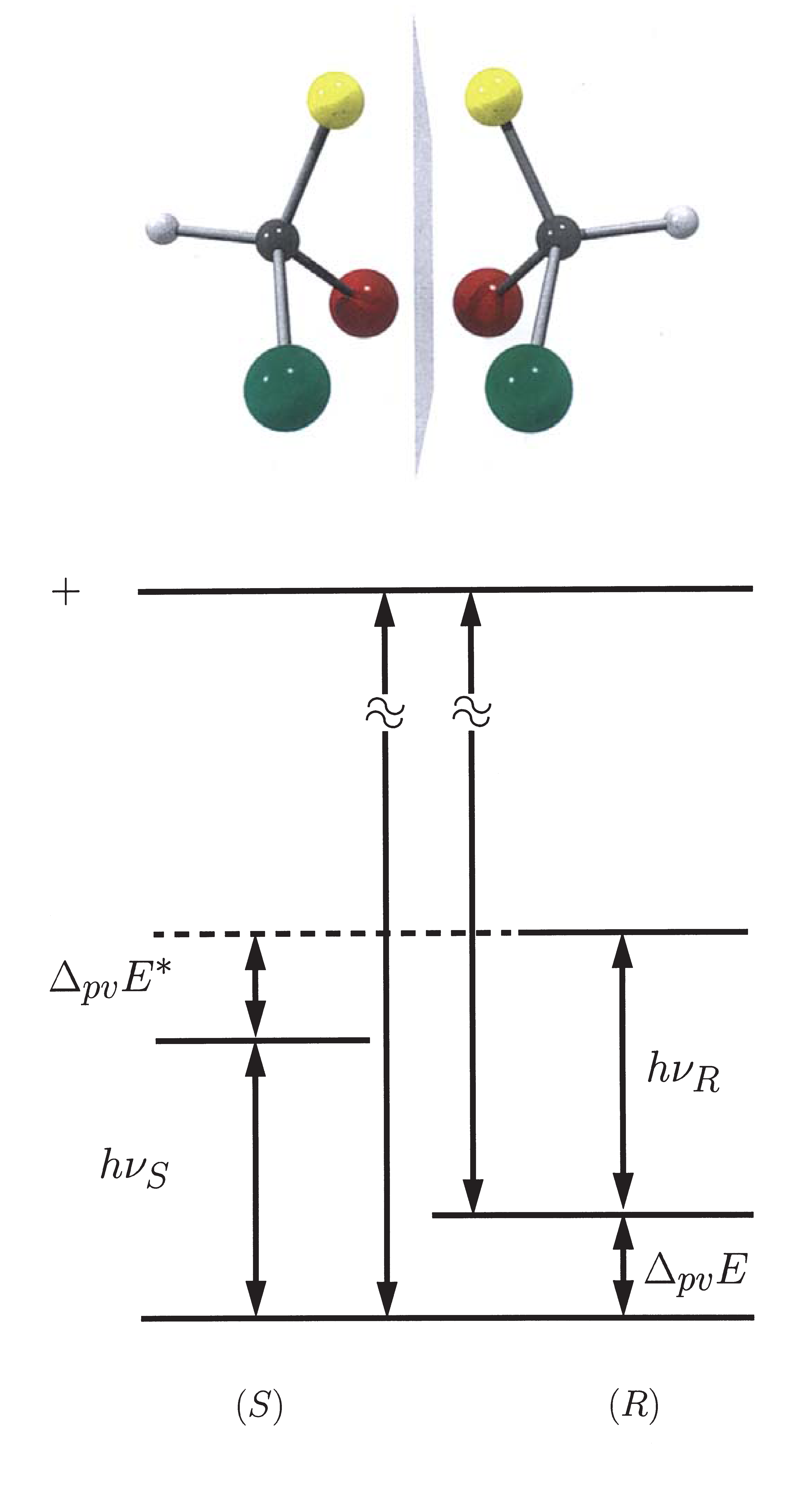
the electroweak interaction is taken into account, a parity violating energy difference ΔPVE
between the two enantiomers (R,S) of a chiral compound is obtained. This energy difference corresponds to
a reaction enthalpy ΔPVH0ø for the stereomutation R ⇔ S.
Theoretical calculations show that this parity violating
energy difference is extremely small, in the order of 10-11 Jmol-1 [1-6]. The parity violating energy
difference has been measured for atoms [7], but its experimental verification for molecules is still missing.
Three different experimental methods have been proposed to measure ΔPVE for molecules.

Image and mirror image of the chiral moloecule CHFBrI
Parity violating energy difference ΔPVE for the ground and ΔPVE* for an excited state