Motivation
Catalysis lies at the heart of chemical industry and is responsible for the production of about 80% of all manufactured products, spanning from basic organic and inorganic chemicals to cosmetics and pharmaseuticals. In 2019, the chemical industry invested $33.9 billion in catalysis, with metal catalysis comprising roughly a quarter of the overall expense. The global demand for catalysis continues to grow, intending to establish new atom- and energy-efficient routes, as well as yield-improved, cost- and energy-saving processes. These processes are expected to solve the present-day risks of a growing population, helping to transit towards a carbon-neutral society, and managing and reducing waste. It is clear that already established routes will not cover the increasing demand, and that we need new, better catalysts.
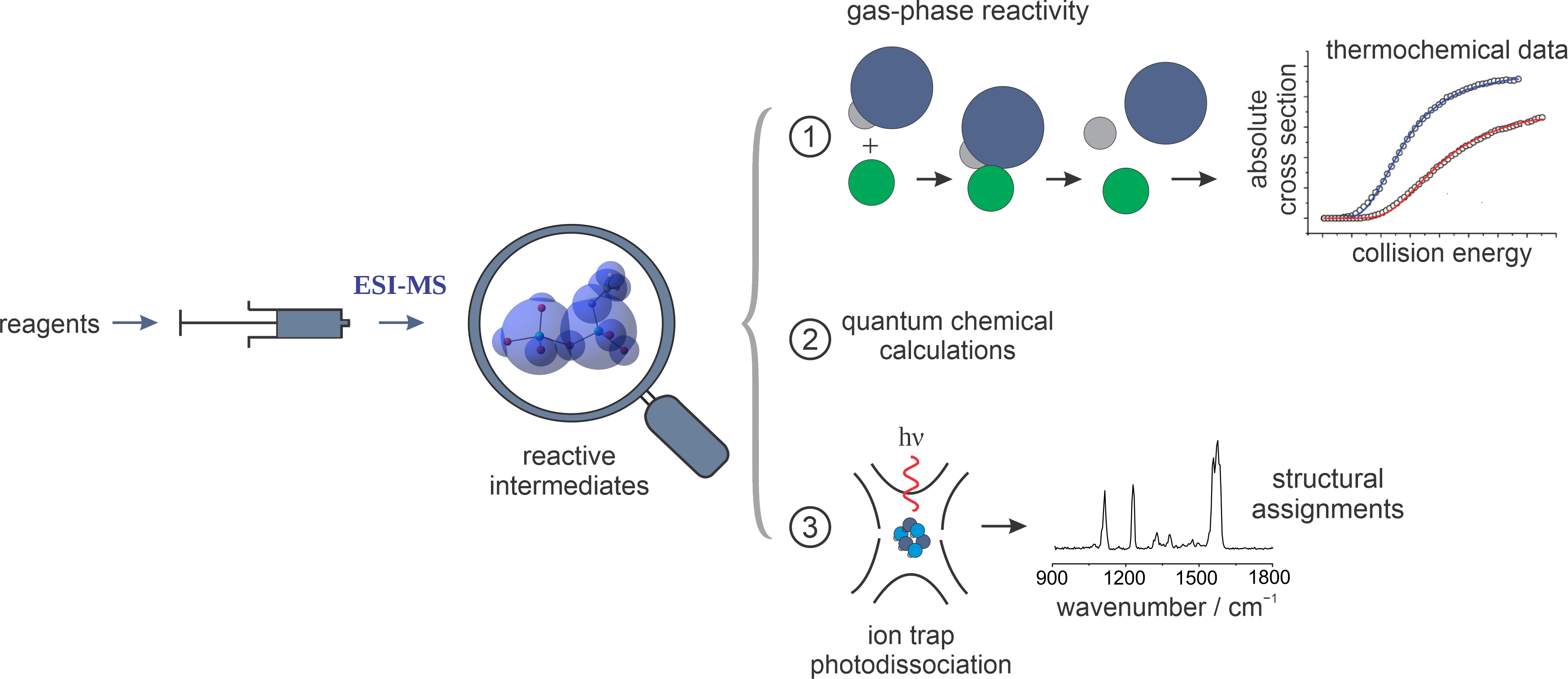
The current approach to better catalysts still involves a trial-and-error process due to an enormous chemical space of metal-ligand combinations and possible reactions that can be investigated. This approach is costly, and is based on the prior knowledge of catalytic reactions. In contrast, our approach is bottom-up, capitalizing on the tools developed for physical chemistry. In our reactivity studies, we develop well-defined molecular models of a reaction at hand, and we combine that with structural investigation of reactant, intermediate, and product species, using spectroscopy and computational chemistry.